

Site Search
Search within product
第736号 2021(R03).12発行
Click here for PDF version
§先人が築いた坂井農場(第2回)
〜120 Years of History - A Look Back at the Transition of Varieties, Fertilizers, etc. as Reflected in the Transcripts
JA Fukui Sakai Farm
前 農場長 長谷川 彰
§土のはなし−第7回
よい土の条件 化学的性質−その2
酸性障害がでる土とでにくい土
Jcam Agri Co.
北海道支店 技術顧問
松中 照夫
§2021年本誌既刊総目次
Sakai Farm built by our predecessors (Part 2)
〜120 Years of History - A Look Back at the Transition of Varieties, Fertilizers, etc. as Reflected in the Transcripts
JA Fukui Sakai Farm
前 農場長 長谷川 彰
III. Showa Era (1st to 20th century, prewar)
大正時代の全国平均収量は290kg/10aで,明治時代末に比べやや増加したものの依然として低水準となっていました。
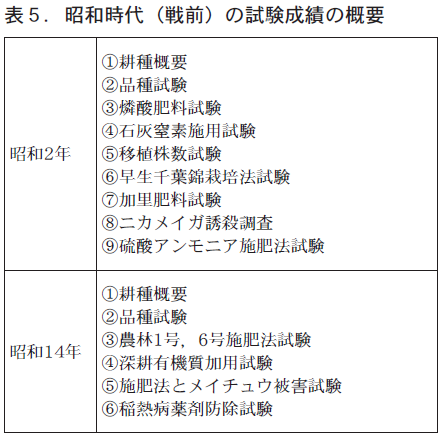
昭和2年も大正時代と同様,水稲単独の取組みで,過燐酸石灰や石灰窒素,硫安,硫酸加里を用いた元肥・追肥試験や害虫防除などきめ細かな試験を実施しています。昭和14年には国で育成された農林1号,農林6号の栽培試験や稲熱病防除試験を実施しています。
昭和16年には,太平洋戦争が始まり,17年に米の全量を国が買い入れする食糧管理法が制定されました。
(1) Outline of variety testing (from local native varieties to government-bred varieties such as Norinbayashi-go 1)
昭和2年の成績書によると,主に福井県立農事試験場から種子を取り寄せ,北陸地域や山形県の本間農場からも入手しています。『大場』『米光』『酒田早生』『赤珍子』『交配25号』『晩3号』など26品種が栽培されています。昭和14年頃には,福井県立農事試験場など県内各地から,県外からは富山県から種子を取り寄せています。『農林1号』『近畿19号』『強新石白』など17品種で在来品種に加え,国等の試験場で交配された品種が多くなっています。昭和16年における福井県の主な品種は,『農林1号』『福井銀坊主』『白珍子』『農林6号』『中生旭』で,『農林1号』『福井銀坊主』で全体の43%を占め,大正時代と比べ一段と品種の集約化が進みました。

(2) Outline of fertilizers, etc. (from soybean meal to chemical fertilizers and mono-fertilizers)
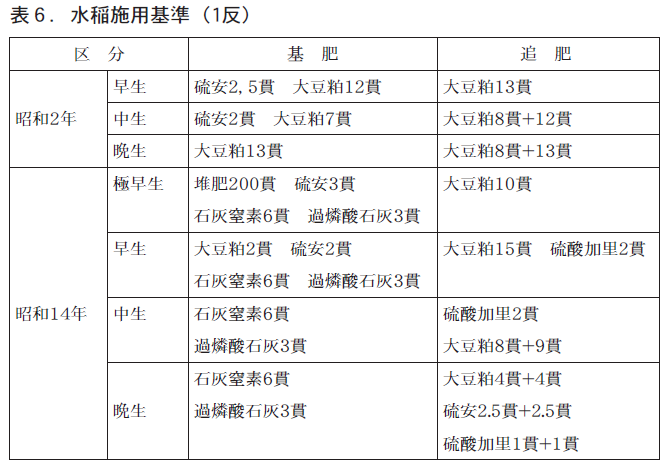
昭和2年の施用基準をみると,苗代肥料(大豆粕,過燐酸石灰,硫安),本田肥料(大豆粕,硫安)が使用されています。
昭和14年頃には,苗代肥料(硫酸加里,硫安,過燐酸石灰),本田肥料(石灰窒素,硫酸加里,硫安,過燐酸石灰,大豆粕)が一般に使用されており,大正時代と比べ化学肥料のウエイトが高くなってきています。
昭和14年の1反あたりの肥料費(中生)は硫酸加里2貫,大豆粕17貫,石灰窒素6貫など合計11円57銭でした。なお,米価は16円35銭/俵で,米収入に占める肥料費の割合は13%と大正13年ごろとほぼ同様でした。
IV. Showa Era (FY21-FY63)
The national average yield from the first to the 20th year of the Showa Era was 300 kg/tract, almost the same level as the 290 kg/tract during the Taisho Era (1912-1926). In addition to weather disasters such as cold damage, shortages of production materials such as fertilizers are thought to have been a major factor.
In addition to variety and fertilizer tests, in FY2013, five years after the end of the war, a fertility test was conducted to clarify the relationship between weather and paddy rice growth, and technical information on growth was provided, which was considerably enhanced compared to the prewar period. In addition, the company has improved seedling cultivation methods by conducting comparative tests of heat-retaining eclectic nurseries and normal nurseries, and has conducted detailed surveys of urea fertilizer, ammonium sulfate, and potassium chloride as well as other methods of ear fertilization.
The national average yield from 1946 to 1965 was 357 kg/10a (353 kg in Fukui Prefecture), a significant increase from the first half of the Showa period (1912-1945).

Unfortunately, the farm's records up to 1973 have since been lost and are unknown.
昭和36年には自立経営を目指した農業基本法が制定され,昭和44年には,コシヒカリなどの良質米の流通を促進するため,自主流通米制度が始まりました。
昭和41年以降も収量は増加し有史以来初めてコメ不足から過剰時代に,昭和44年から試験的に転作が実施されました。
昭和49年には,早生品種こしにしきの機械化対応や低コストを目的とした直播栽培,良食味品種のコシヒカリの作付け拡大を目指し原種確保・提供に努め時代の変化が感じられます。昭和53年からは,本格的な生産調整・水田利用再編対策が実施されました。増収技術の追求から省力と品質・食味重視と大きく転換してきました。
昭和63年には,早生のフクヒカリ,中生のコシヒカリなど早場米,良質米の多収試験や倒伏軽減対策に加え,転作面積の拡大に対応し麦の奨励品種試験や米の他用途利用を念頭に多収品種の試作に取り組んでいます。
(1) Outline of variety testing (from No.1 to Hounenwase and Koshihikari)
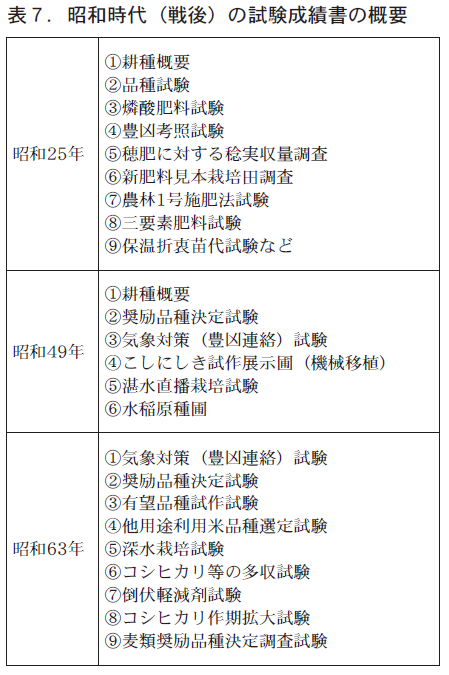
この時代前半の品種試験は収量,その後は収穫時期や味等に重点が置かれています。昭和26年の試験は,『農林1号』などをベースに『農林32号』『関東41号』『近畿33号』『千本旭』など14品種で実施されています。当時の福井県の主な品種は『農林1号』で作付面積の21%を占めています。
Around 1974, "Hounenwase," "Koshihikari," and "Kimpa" were the standard varieties, and "Echinan 111," "Echinan 110," and "Tokai 41" were grown in trials, with many of these varieties bred at the Fukui Agricultural Experiment Station, a designated national experimental site. The main varieties grown in Fukui Prefecture in 1973 were "Hounenwase," "Kimpa," and "Koshihikari," with "Hounenwase" accounting for about 501 TP3T of the total.
In 1988, five varieties, including "Echigo-Nan 140," "Echigo-Nan 143," and "Yamahikari," were planted, with the early variety "Fukuhikari" and the late variety "Nihonbaru" as the standard. The main varieties in Fukui Prefecture in 1983 were "Koshihikari," "Fukuhikari," and "Nihonbaru," with "Koshihikari" accounting for approximately 371 tons of the total. Koshihikari" was a variety that met the needs of the times, from increased food production to good taste. In 1988, the price of Koshihikari rice for voluntary distribution (Fukui Prefecture) was 22,300 yen per bale, which was higher than the government rice price.
(2) Outline of fertilizers, etc. (from mono-fertilizers to compound fertilizers and advanced compound fertilizers)
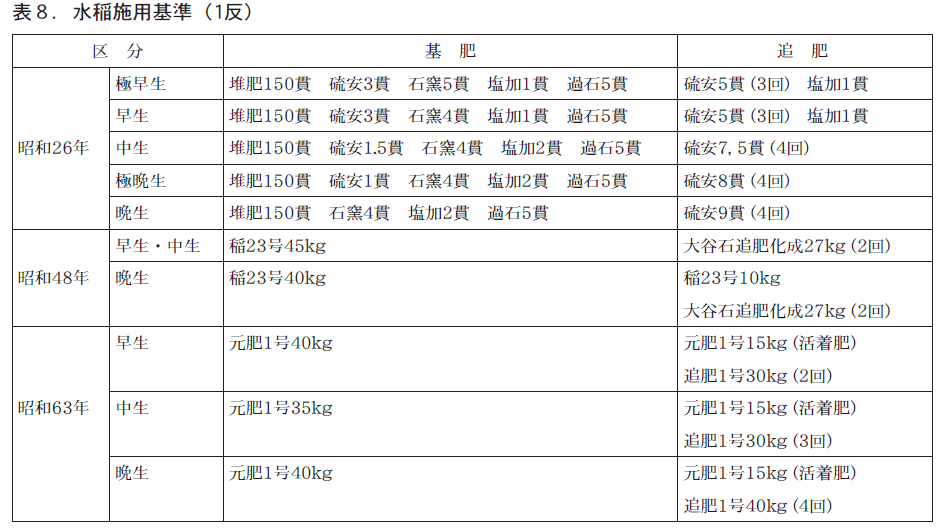
昭和26年の施用基準は,苗代肥料(堆肥,過燐酸石灰,硫安,塩化加里),本田肥料(堆肥,過燐酸石灰,硫安,塩化加里,石灰窒素)で,新肥料試験として,固形肥料,粒状石灰窒素,尿素などが使用されています。
Around 1973, rice field fertilizers (rice No. 23 and Oyaishi composite fertilizer) were used, and there was a shift from single fertilizers to compound fertilizers. In 1988, the first and second fertilizers were used, and the Koshihikari rice was fertilized about 12 kg/10a in N content and three times with ear fertilizer. The reason for the much higher amount of N applied to Koshihikari than today is thought to be that the fertilizer absorption and growth characteristics of Koshihikari were not fully understood, and that yield was still more important than quality and taste in the face of high prices.
Fertilizers were mainly highly chemical fertilizers, and today's bases were established around this time. In addition, the names and packages of fertilizers were standardized throughout the prefecture, and each manufacturer was able to demonstrate its own originality in the ammonium sulfate and ammonium chloride types, etc.
Fertilizer costs in 1973 and 1988 were 3,459 yen/10a and 10,605 yen/10a, respectively, according to the Annual Report of Prefectural Crop Statistics. The price of rice increased from 10,390 yen per bale in 1973 to 16,743 yen per bale in 1988. The fertilizer cost as a percentage of rice income in 1988 was about 81 TP3T.
No Soil - No. 7
よい土の条件 化学的性質−その2
酸性障害がでる土とでにくい土
Jcam Agri Co.
北海道支店 技術顧問
松中 照夫
Last month, I pointed out that the appropriate pH (pH measured using pure water H2O) for good soil for crop production is in the range of 5.5 to 6.5. The reason why the appropriate range is slightly on the acidic side is that the soil in Japan is subject to acidification due to abundant rainfall, and crops suitable for such conditions are grown in Japan. He also pointed out that, of the factors that cause soil acidification and adversely affect crop growth, aluminum (Al) is the factor that has the greatest adverse effect.
However, there are various kinds of soils that are highly acidic with a pH lower than 5.0 but do not cause serious damage to crops. This month, we will consider why this is the case.
1. soil that allows corn to grow vigorously even in highly acidic conditions
First, look at Figure 1. This is the result of corn cultivation using two types of soil derived from volcanic ash widely distributed in Japan (commonly known as volcanic ash soil, or more correctly, black box soil).

図1で左の二つは,わが国の代表的な黒ボク土で,アロフェン質黒ボク土とよばれる土である(以下では,A黒ボク土と略)。明確な結晶構造をつくらない粘土鉱物(アロフェンやイモゴライトといった粘土鉱物)を持っていることからつけられた名前である。一方,右の二つは東北地方や北海道,さらに本州では日本海側などに分布する特殊な黒ボク土で,非アロフェン質黒ボク土といわれる土である(以下では,N黒ボク土と略)。この黒ボク土の主要な粘土鉱物はアロフェンではなく,明確な結晶構造を持つ粘土鉱物であることが大きな特徴である。
The pH of the A black soil was 4.8 and that of the N black soil was 4.5, making them highly acidic soils. We compared the growth of corn plants in these soils with and without acidification with calcium carbonate (calcium carbonate), an alkaline material, after supplying sufficient lime superphosphate as a phosphorus material.
If the soil is highly acidic, as these two A black box soils are, corn growth should deteriorate unless the soil is acidified. However, strangely enough, there was no significant difference in the growth of corn in the left two A black soil, regardless of whether the soil was acidified or not. On the other hand, in the case of the two N black box soils on the right, the growth of corn plants was greatly suppressed without acidification. In other words, there were two types of soils: one in which acid damage appeared in the crop even when the soil was acidified (in the case of the two N black box soils on the right of Fig. 1), and the other in which this did not occur (in the case of the two A black box soils on the left of Fig. 1). Why is this?
2. exchange acidity (y1) was different
通常,土のpHは純水(H2O)を使って測定している。しかし,もう一つの測定方法として,塩化カリウム(KCl)溶液を用いる場合がある。そのときはpH(KCl)と表示する。
The idea of using potassium chloride solution to measure pH came from Gintaro Daikubara, who was the first in the world to research methods of improving soil acidity. He believed that soil acidification adversely affects crops because of aluminum ions dissolved in soil moisture (soil solution), and that the degree of adverse effect is determined by the amount of exchangeable aluminum retained in the soil. As a method to measure this, he devised an ion-exchange method in which the exchangeable aluminum retained in the soil was ion-exchanged with potassium ions in a potassium chloride solution and released into the solution.
土から塩化カリウム溶液に放出されたアルミニウムイオンは,溶液のH2Oと次々に反応して水素イオンをつくりだす。このため,水素イオン濃度が濃くなって酸性度が強まる。この強まった酸性度をアルカリ性の水酸化ナトリウム(苛性ソーダ)溶液で中和し,その中和に必要な水酸化ナトリウムの量(ミリリットル=㎖)で,間接的にアルミニウムイオンを測定しようとした。この㎖値を交換酸度とよび,記号をy1(ワイワン)とした。
The y1 values of the two black granite soils used in the experiment shown in Figure 1 were 4.4 for A-Black soil and 28.0 for N-Black soil, which is significantly different from the y1 value of A-Black soil. In other words, the A soil was originally low in exchangeable aluminum, the substance responsible for acid damage, while the N soil had nearly seven times more than the A soil.
Therefore, it can be understood that corn, which is relatively tolerant to acidity, did not suffer from growth failure with the amount of exchangeable aluminum as low as that of A black soil, even if the pH was very acidic. On the other hand, if the amount of exchangeable aluminum is high, as in the case of N black soil, the amount of aluminum ions in the soil solution will increase and cause acid damage to the roots if the soil is not acidified.
3. why exchange acidity (y1) varies from soil to soil
The reason that corn could grow without acidification even though the soil is highly acidic is because the A black box soil could not retain much exchangeable aluminum. The question is why.
これにはまことに不思議なことなのだが,土が持っているマイナスやプラスの静電気(負荷電と正荷電)の性質が深く関わっている(詳細はこの連載で改めて述べる)。
A黒ボク土が持つ負荷電や正荷電は,周りの土壌溶液のpHによって大きく変化する(これを変異荷電という)。この性質をもつ負荷電は,土が酸性に傾くとそれ以上酸性化しないように作用し,プラスの電気を帯びた水素イオンが土壌溶液へ放出されないよう自分の負荷電に静電気的にひきつけて保持した状態を維持する。そうなると,負荷電は水素イオンでふさがれたままなので,空いた負荷電がない。プラスの電気をもつアルミニウムイオンは土の負荷電に引きつけられて交換性アルミニウムとなる。しかし,そのための空いた負荷電がないA黒ボク土では,交換性アルミニウムが安定して保持される場所がなく,その結果として交換酸度(y1)が小さくなる。
On the other hand, the N-black soil does not have the same properties as the A-black soil and functions as a loading charge at all times. Therefore, when aluminum ions with a positive charge approach the soil, they are attracted to and retained by the loading charge, exchanging ions with hydrogen ions held in the loading charge. Therefore, exchangeable aluminum can exist stably in N-black soil, and the amount of exchangeable aluminum is increased, resulting in a large exchange acidity (y1).
4. the importance of measuring exchange acidity (y1)
Japanese soil is prone to acidification. Therefore, it is often pointed out that we must prevent acidification by giving charcoal every year. This emphasis on the need to prevent acidification has led to the careless application of calcium carbonate to soil without any soil diagnosis, resulting in the creation of soil with a high pH level. To determine whether a soil needs special attention with regard to acidification, y1 should be measured.
N-black soil susceptible to acid injury is considered to have a y1 value of 5 or higher. The criteria for determining whether a soil is susceptible to acid injury vary from crop to crop because each crop has a different tolerance to acidity. Therefore, it is difficult to generalize the criteria. However, it is important to add y1 to soil diagnosis as well as pH measurement.
2021年本誌既刊総目次
<1月号>
§「更なる新しい常態」の創造に向けて
ジェイカムアグリ株式会社
営業統括本部長 田代 教昭
§春まきタマネギ栽培における肥効調節型肥料の利用
福島県農業総合センター
作物園芸部野菜科
副主任研究員 横田 祐未
§行灯仕立てアサガオ鉢の購入後施肥方法と長尺仕立てアサガオポット苗による新たな植物装飾の提案
東京都農林総合研究センター
江戸川分場
田旗 裕也
<2月・3月合併号>
§トマト,メロンの紐栽培における肥料袋の投入箇所と培地の太陽熱消毒について
元 岡山大学大学院 自然科学研究科
桝田 正治
今野 裕光
§<産地レポート>
兵庫県JA丹波ひかみ「山の芋」栽培で活躍する緩効性肥料「CDU化成S555」について
ジェイカムアグリ株式会社 西日本支店
<4月号>
§湛水条件下の水田土壌の可給態リン酸の温度反応とその推定
東北大学大学院農学研究科
附属複合生態フィールド教育研究センター
教授 西田 瑞彦
§気候変動緩和と肥効調節型肥料
千葉大学大学院 園芸学研究院
名誉教授 犬伏 和之
<5月号>
§果樹の盛土式根圏制御栽培における肥培管理技術
栃木県農業試験場 研究開発部果樹研究室
(現 栃木県農業大学校)
高橋 優太郎
§土のはなし−第1回 よい土とはどんな土か
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
<6月号>
§加里不足圃場への加里肥料増施による大麦収量性の改善
富山県農林水産総合技術センター
農業研究所
南山 恵
§土のはなし−第2回 よい土の条件
物理的性質−その1 根を支える土の厚み
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
<7月号>
§被覆肥料「Jコート」の水稲に対する全量基肥施用の効果と被膜崩壊性
秋田県北秋田地域振興局農林部
主査 松田 英樹
(元所属 秋田県農業試験場)
§土のはなし−第3回 よい土の条件
物理的性質−その2
土の硬さはどのようにして決まるのか
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
<8月・9月合併号>
§水稲多収品種「ミズホチカラ」に対する育苗箱全量施肥による省力栽培と大規模経営体における導入効果
熊本県農業研究センター生産環境研究所
研究参事 柴山 豊
(現在 熊本県県北広域本部阿蘇地域振興局)
§土のはなし−第4回 よい土の条件
物理的性質−その3 断面でわかる排水の良否
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
<10月号>
§トマト水疱症発生の品種間差異は地上部重と地下部重の割合が関与する
福島大学農学群食農学類
深山 陽子
§土のはなし−第5回 よい土の条件
物理的性質−その4
適度に水を保持し排水もよい土とは
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
<11月号>
§ブロッコリー栽培におけるマイクロロングトータルのセル苗施用効果
千葉県農林総合研究センター
水稲畑地園芸研究所 東総野菜研究室
竹内 大造
(現:海匝農業事務所 改良普及課)
§先人が築いた坂井農場(第1回)
〜120年の歴史−成績書から振り返る品種・肥料等の変遷〜
JA福井県 坂井農場
前 農場長 長谷川 彰
§土のはなし−第6回 よい土の条件
化学的性質−その1 土の酸性度(pH)
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
<12月号>
§先人が築いた坂井農場(第2回)
〜120年の歴史−成績書から振り返る品種・肥料等の変遷〜
JA福井県 坂井農場
前 農場長 長谷川 彰
§土のはなし−第7回 よい土の条件
化学的性質−その2
酸性障害がでる土とでにくい土
ジェイカムアグリ株式会社 北海道支店
技術顧問 松中 照夫
§2021年本誌既刊総目次